ELI-002
Our lymph node-targeted mKRAS cancer vaccine
Our Amphiphile (AMP)-powered lead candidate, ELI-002, generates a robust immune response against mutant Kristen rat sarcoma (mKRAS), with the potential to offer patients a stronger fight against the most aggressive cancers.
Preliminary data have shown that ELI-002 produces a potent mKRAS-specific T-cell response, including both CD4+ and CD8+ populations, that correlates with tumor biomarker responses, potentially leading to improved clinical outcomes.
The KRAS opportunity
Seven KRAS driver mutations are present in 25% of all solid tumor cancers and are found in 88% of pancreatic ductal adenocarcinoma (PDAC) cases, 36% of colorectal cancer (CRC) cases, and 25% of non-small cell lung cancer (NSCLC) cases. PDAC accounts for more than 450,000 deaths per year globally and is the seventh leading cause of cancer deaths.
There is an urgent need for innovative immunotherapies that address this historically “undruggable” mutation. Immunotherapies that precisely target mKRAS-driven cancers have the potential to improve therapeutic outcomes and increase patient survival rates. ELI-002 is a novel cancer vaccine that could harness the power of lymph nodes to elicit potent immune responses and target mKRAS with greater potency and precision.
Engineered for modularity
With its structurally novel composition, ELI-002 has the potential to become a universal mKRAS therapy with the ability to treat hundreds of thousands of patients with mKRAS-driven cancers.
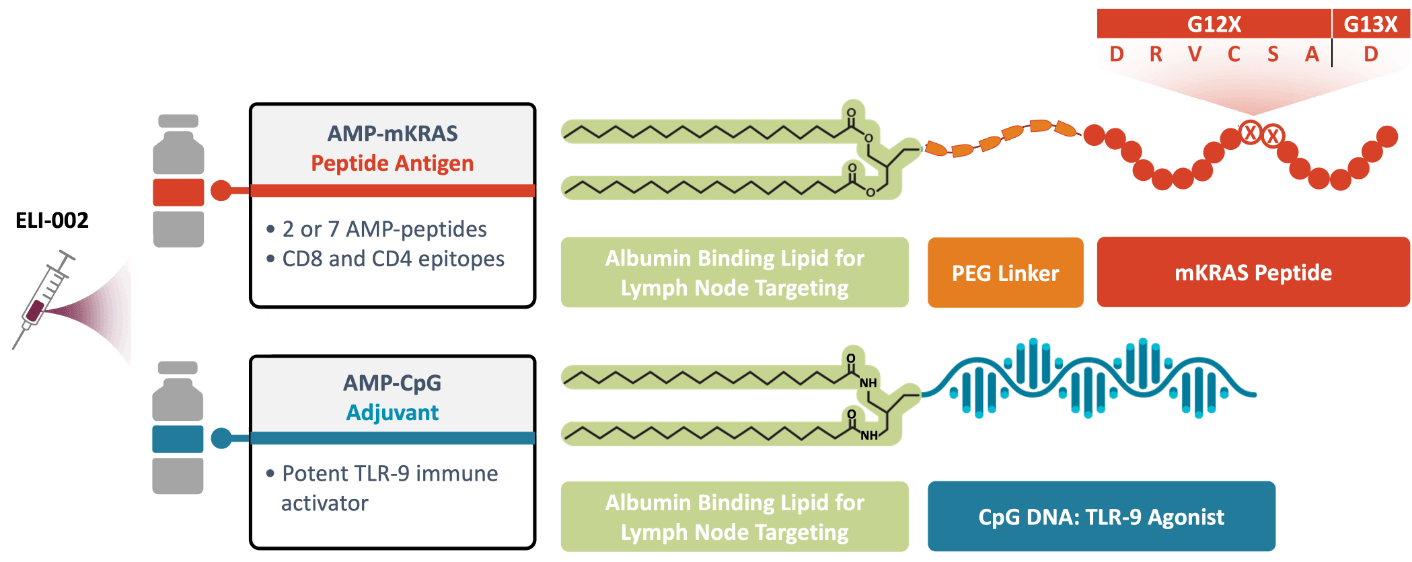
ELI-002 is comprised of 2 powerful components that are built with our AMP platform:
AMP-CpG: Our proprietary immune activator. A universal AMP-modified CpG adjuvant designed for application in a variety of therapies and indications.
AMP-mKRAS-Peptides: AMP-mKRAS peptide antigens that have the potential to target a broad spectrum of KRAS mutations.
Utilizing the power of the AMP technology, both components are targeted to the lymph nodes, where they could enhance the action of key immune cells, generating an overall robust tumor-specific immune response.
AMPLIFY clinical program
We are studying 2-peptide (2P) and 7-peptide (7P) formulations of ELI-002 in patients with mKRAS-driven tumors who are at a high risk for relapse, following standard surgery and chemotherapy.
AMPLIFY-201: A phase 1a dose-escalation study evaluating the safety, immunogenicity, and antitumor activity of ELI-002 2P, a 2-peptide formulation, in patients with G12D or G12R KRAS mutations with a high risk of relapse. Promising preliminary results from AMPLIFY-201 demonstrate the potential of ELI-002 to generate potent T-cell responses that correlate with clinical outcomes. Median tumor biomarker reduction/clearance was -86.9% vs -1.0% in above vs below median T-cell responders. The median relapse-free survival was not reached compared with 3.91 months in above vs below median T-cell responders (hazard ratio, 0.14; 95% CI, 0.031-0.610; P=0.0134).
AMPLIFY-7P: Enrollment is underway for AMPLIFY-7P, a phase 1/2 trial designed to study ELI-002 7P, a 7-peptide formulation armed to stimulate an immune response against the 7 most common KRAS mutations that drive 25% of solid tumors. ELI-002 7P will be evaluated in a randomized phase 2 study in patients with mKRAS-driven pancreatic cancer. Patients will be randomized 2:1 to receive ELI-002 or undergo observation. The observed patients may cross over to ELI-002 if relapse is confirmed on an imaging scan. Additional trials are in development for patients with mKRAS-driven cancers, such as CRC and NSCLC.
ELI-002 therapeutic focus
Pancreatic ductal adenocarcinoma (PDAC)
PDAC is the most common type of pancreatic cancer and the fourth leading cause of cancer-related deaths worldwide. Genetic mutations found in PDAC are primarily linked to the oncogene KRAS and tumor suppressor genes.1 Immunotherapy is a promising treatment approach to PDAC that leverages the immune system for antitumor activity.2
Additional mKRAS-driven cancers with high unmet need, such as CRC and NSCLC, are therapeutic areas of interest for future studies.
ELI-002 publications and presentations
References
- Sally Á, McGowan R, Finn K, Moran BM. Current and Future Therapies for Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2022;14(10):2417. Published 2022 May 13. doi:10.3390/cancers14102417
- Beatty GL, Eghbali S, Kim R. Deploying Immunotherapy in Pancreatic Cancer: Defining Mechanisms of Response and Resistance. Am Soc Clin Oncol Educ Book. 2017;37:267-278. doi:10.1200/EDBK_175232