AMP platform
Targeting the lymph nodes to amplify the immune response
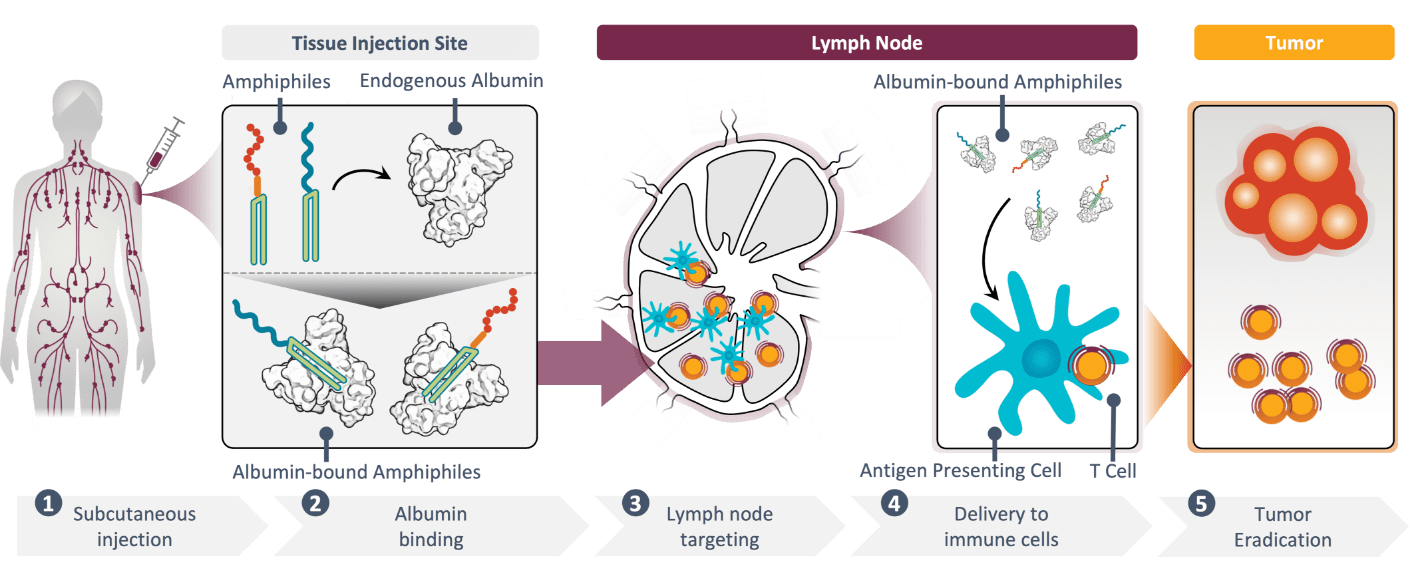
Our Amphiphile (AMP) platform, developed at the Massachusetts Institute of Technology (MIT), delivers therapeutic payloads directly to the lymph nodes to enhance the cancer-fighting mechanisms of the immune system.
By delivering cancer immunotherapies to the center of the immune response, our platform is intended to optimize the natural ability of the lymph nodes to educate, activate, and amplify cancer-specific T cells. Engineered to coordinate immunity in these uniquely potent sites, our platform is built to amplify the magnitude, potency, quality, and durability of the immune response to drive antitumor activity.
We are committed to applying this lymph node-targeting approach across a range of vaccines, immunomodulators, and adjuvants—training the immune system to put the best cells forward to fight a broad spectrum of cancers.
Our AMP platform has demonstrated the ability to:
- Prevent detrimental payload delivery to systemic circulation (i.e., immunologically irrelevant or tolerizing sites)
- Direct payloads into lymphatics to promote lymph node delivery
- Preserve structural integrity and activity of payloads by preventing degradation
- Promote lymph node retention
- Promote direct payload delivery to key educational immune cells
- Activate and amplify cancer-specific immune cells (in lymph nodes)
Our AMP-powered immunotherapies have demonstrated the ability to:
Activate immune mechanisms
Improve the ability of T cells to find and enter tumors throughout the body
Expand and activate T-cell response
Improve T-cell persistence
Promote antitumor T-cell function
Reduce the risk of resistance mechanisms
Molecule size matters
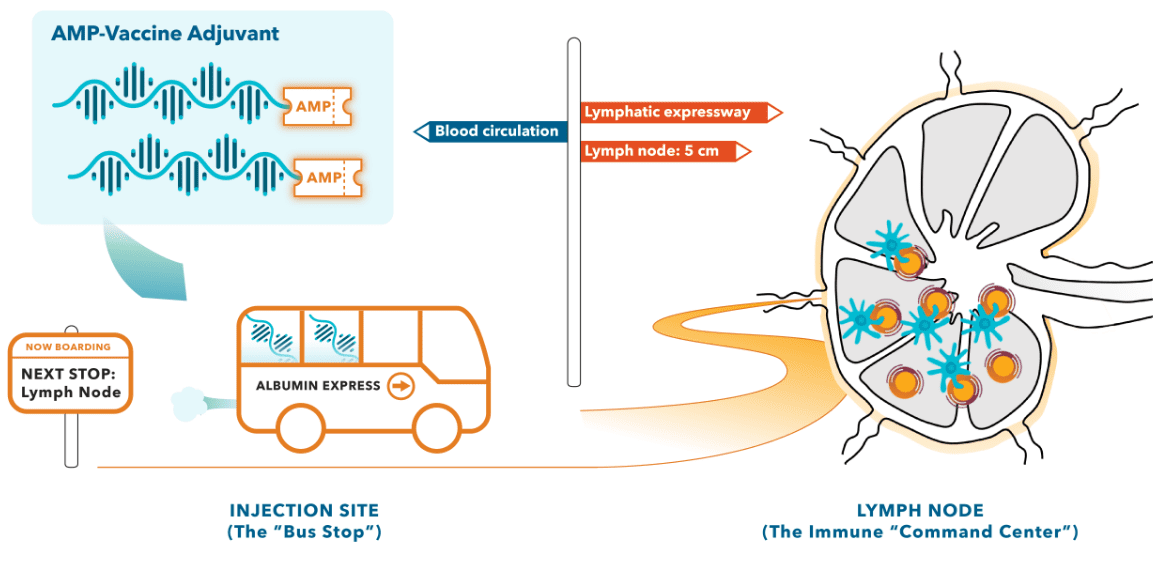
Immune responses are orchestrated by key immune cells in the lymph nodes. But for many therapies, getting to these critical sites is far from certain. Small vaccine components and other immunomodulators easily pass through the blood vessel walls at the injection site.1 As a result, they are rapidly flushed away into the systemic circulation, preventing access to the lymph nodes. Often, this results in a failure to realize the full potential of the immune response, or worse yet, leads to the development of dangerous toxicities at other sites in the body.
However, larger proteins, such as albumin, almost exclusively travel from the injection site into the lymph vessels, promoting direct delivery to immune cells in the lymph nodes.1 We believe that lymph node-targeting made possible by utilizing the natural trafficking patterns of larger molecules holds great promise for enhancing immunologic responses and therapeutic efficacy. Built around this concept, our AMP platform leverages albumin “shuttling” by tethering therapeutic payloads to endogenous albumin at the injection site for direct delivery to the lymph nodes.
See how we are harnessing our AMP platform to defeat difficult-to-target cancers
AMP platform publications and presentations
Reference
- Steinbuck MP, Seenappa LM, Jakubowski A, McNeil LK, Haqq CM, DeMuth PC. A lymph node–targeted amphiphile vaccine induces potent cellular and humoral immunity to SARS-COV-2. Science Advances. 2021;7(6). doi:10.1126/sciadv.abe5819